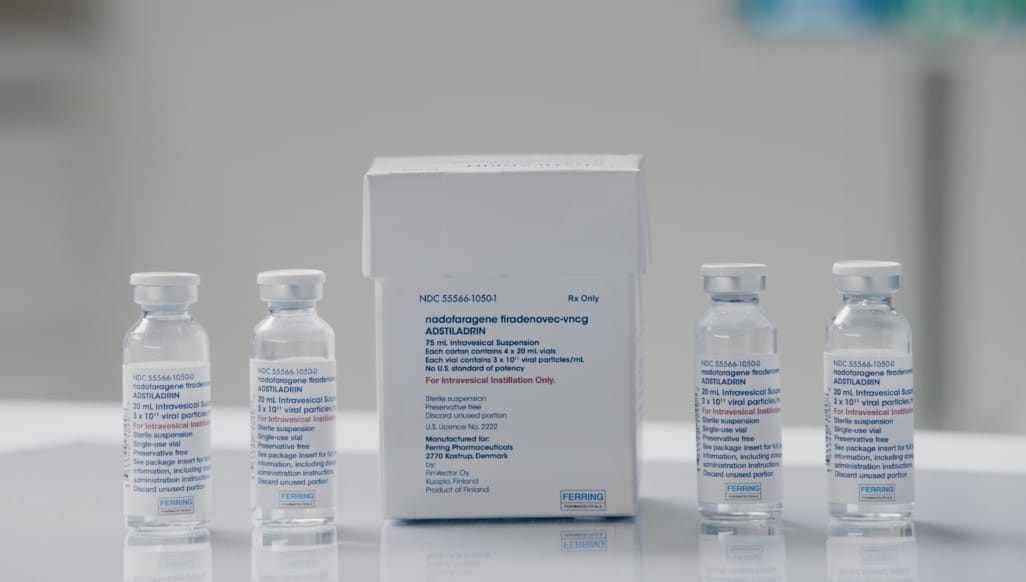
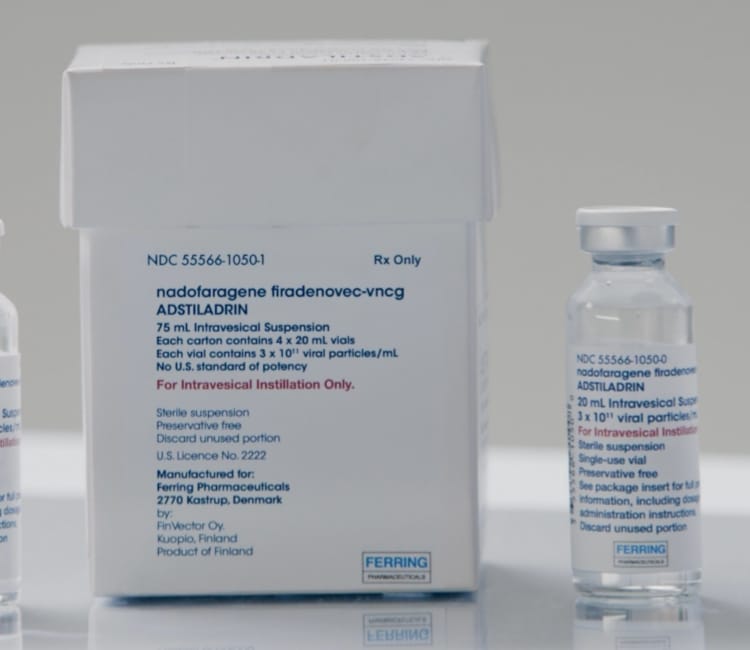
- The recommended dose of ADSTILADRIN (nadofaragene firadenovec-vncg) is 75 mL at a concentration of 3 x 1011 viral particles/mL
- Premedication with an anticholinergic is recommended before each instillation of ADSTILADRIN

Dosing
ADSTILADRIN Is an Intravesical Instillation Given Once Every 3 Months
ADSTILADRIN offers seamless integration into your urology practice, reducing the treatment burden

Quarterly dosing:
once every 3 months

Familiar intravesical instillation

Administered
in your urology office

No required reconstitution or dilution
No Bacillus Calmette-Guérin (BCG) coadministration
is required with ADSTILADRIN
ADSTILADRIN in Practice
Neal Shore, MD, FACS, a clinician from the ADSTILADRIN clinical trials, shares important information about implementing ADSTILADRIN in your office.

Related resource Download
For more on dosing as well as administration details, please download the Instructions for Use and see full Prescribing Information
Sign Up to Receive Information and Updates on ADSTILADRIN (nadofaragene firadenovec-vncg)
Form ToggleReference: ADSTILADRIN. Package insert. Ferring Pharmaceuticals, Inc; August 2024.
Important Safety Information
INDICATION
ADSTILADRIN is a non-replicating adenoviral vector-based gene therapy indicated for the treatment of adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS: ADSTILADRIN is contraindicated in patients with prior hypersensitivity reactions to interferon alfa or to any component of the product.
WARNINGS AND PRECAUTIONS:
- Risk with delayed cystectomy: Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. If patients with CIS do not have a complete response to treatment after 3 months or if CIS recurs, consider cystectomy.
- Risk of disseminated adenovirus infection: Persons who are immunocompromised or immunodeficient may be at risk for disseminated infection from ADSTILADRIN due to low levels of replication-competent adenovirus. Avoid ADSTILADRIN exposure to immunocompromised or immunodeficient individuals.
DOSAGE AND ADMINISTRATION: Administer ADSTILADRIN by intravesical instillation only. ADSTILADRIN is not for intravenous use, topical use, or oral administration.
USE IN SPECIFIC POPULATIONS: Advise females of reproductive potential to use effective contraception during ADSTILADRIN treatment and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during ADSTILADRIN treatment and for 3 months after the last dose.
ADVERSE REACTIONS: The most common (>10%) adverse reactions, including laboratory abnormalities (>15%), were glucose increased, instillation site discharge, triglycerides increased, fatigue, bladder spasm, micturition (urination urgency), creatinine increased, hematuria (blood in urine), phosphate decreased, chills, pyrexia (fever), and dysuria (painful urination).
You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.FDA.gov/medwatch or call 1-800-332-10881-800-332-1088. You may also contact Ferring Pharmaceuticals at 1-888-FERRING1-888-FERRING.
Please see full Prescribing Information for ADSTILADRIN.